Hematopoietic stem cells (HSCs) and downstream lineage-biased multipotent progenitors (MPP) tailor blood production. Recent lineage tracing analyses revealed MPPs to be major functional contributors to steady-state hematopoiesis. However, we are still lacking a precise resolution of lineage differentiation trajectories and cellular heterogeneity in the MPP compartment. We study cell fate decision and lineage specification mechanisms in HSCs and MPPs to modulate lineage output in disease and aging context for therapeutic purposes.
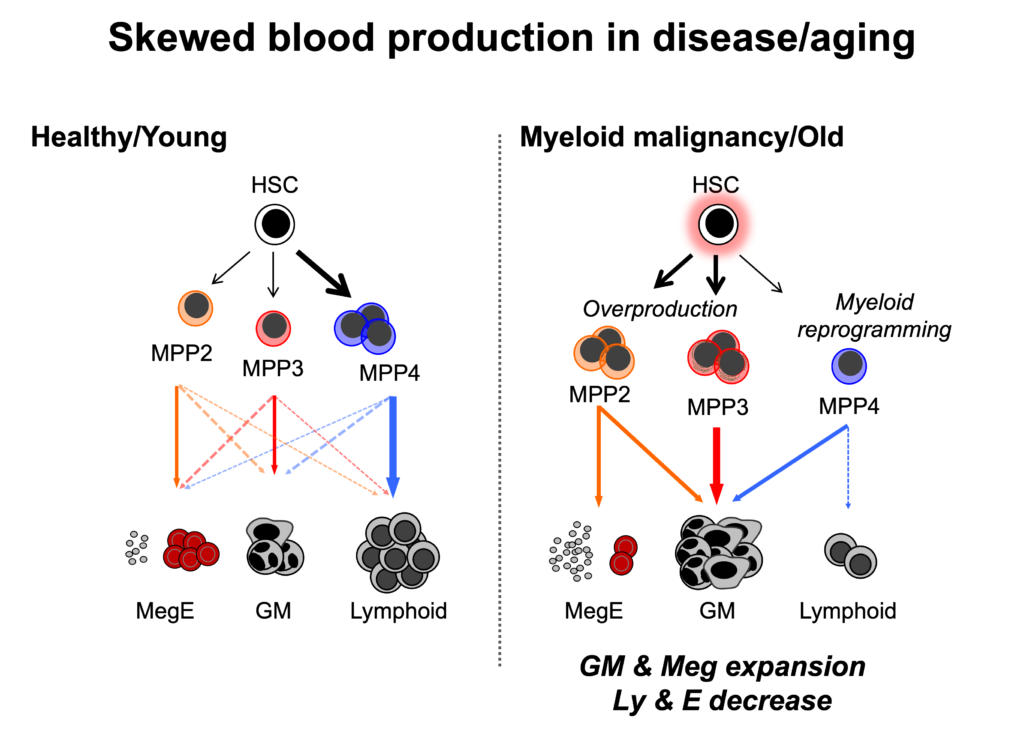
Previously, megakaryocyte (Meg)-biased MPP2 and granulocyte/macrophage (G/M)-biased MPP3 were identified and characterized as novel myeloid-biased MPP subsets. They are functionally distinct from the lymphoid-primed MPP4 and are geared towards the preferential production of myeloid cells with low contribution to the lymphoid lineages. While being much smaller compartments than MPP4 at steady-state, MPP2 and MPP3 are transiently expanded in regenerative conditions and are essential for rebuilding the myeloid lineage.
Blood production skews towards myeloid lineage upon aging and myeloid malignancies with expanded MPP2 and MPP3. Production of G/M or Meg increases while lymphoid and erythroid (E) production decreases, resulting in higher risk of infection, stroke, heart attack, anemia, etc. in the elderly and various complications in myeloid leukemia patients. Therefore, our ultimate goal is to modulate this skewed lineage output to rebalance blood production.
Three major interests:
Lineage priming mechanisms in HSCs
Previously, I showed that high Wnt and low Notch signaling activities make HSCs produce myeloid-biased MPP2 and MPP3, especially G/M-primed MPP3 subset. Signaling pathways functioning in priming HSCs towards lymphoid or erythroid lineage are not well understood nor the interplays between different signaling pathways in HSCs. We are trying to understand the signaling mechanisms of HSC lineage priming as currently focusing on NFkB signaling.
G/M versus E priming in MPP3
MPP3 has two distinct subsets based on endoplasmic reticulum (ER) volume and FcgR expression. ERhigh/FcgR+ MPP3 is a G/M primed MPP3 subset with higher cytokine secretory activity serving as a reservoir for rapid granulocyte macrophage progenitor (GMP) production. ERlow/FcgR– MPP3 represents the multipotent nature of MPP3 with erythroid potential. ERlow/FcgR– MPP3 gives rise to ERhigh/FcgR+ MPP3 but not vice versa, indicating ERhigh/FcgR+ MPP3 is a transitional population towards GMP. What regulates the transition from ERlow/FcgR– MPP3 to ERhigh/FcgR+ MPP3 is under active investigation in the lab focusing on Gata2 and unfolded protein response (UPR) signaling.
Meg versus E priming in MPP2
MPP2 preferentially produces megakaryocytes while maintaining other lineage potentials like erythroid lineage in permissive conditions. Thus, re-wiring lineage output of MPP2 from megakaryocyte to erythrocyte can be beneficial in the elderly and cancer patients such as myelofibrosis. The presence of Meg-biased HSCs and Meg-biased MPP2 generated from HSCs suggests that MPP2 would need to shut down the Meg program to be able to produce erythroid cells. Although functional antagonism between master transcription factors of lineage specification is one critical determinant of fate decision, megakaryocyte and erythrocyte share master regulators in their lineage specification. This indicates that other players function to shut down the Meg program and turn on the erythroid program in MPP2. We are currently focusing on the epigenetic regulator Asxl1 mutation to investigate the underlying mechanisms in MPP2 lineage output.

